Chiral proton-transfer shuttle catalysts for carbene insertion reactions
Abstract
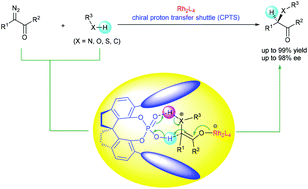
Transition metal-catalyzed carbene insertion into X–H bonds (X = N, O, S, and C) represents a typical carbene transfer reaction and has been widely used in organic synthesis. The enantioselectivity-determining step in some of these insertion reactions is the proton transfer of active intermediates such as ylides, metal enolates, or free enols. Since most of the traditional chiral transition metal catalysts tend to dissociate from these active intermediates and cannot be involved in the proton-transfer step, enantiocontrol of these insertion reactions has long been a challenging task. Since 2011, we have developed chiral spiro phosphoric acids as chiral proton-transfer shuttle (CPTS) catalysts, which have been proven to be efficient catalysts for the proton transfer of active intermediates in carbene insertion reactions. Upon combining with achiral dirhodium catalysts, the CPTS catalysts accomplish highly enantioselective insertions of N–H, S–H, and C–H bonds. Herein, a number of important chiral building blocks, including α-amino acid derivatives, α-amino ketones, α-thioesters, and α,α-diaryl acetates, were prepared with high yields and high enantioselectivities through these insertion reactions.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...