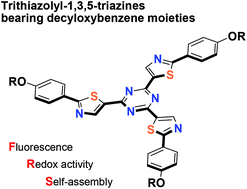
Trithiazolyl-1,3,5-triazines bearing decyloxybenzene moieties: synthesis, photophysical and electrochemical properties, and self-assembly behavior†
Abstract
We report the synthesis, photophysical properties, redox characteristics, and self-assembly behavior of disk-shaped trithiazolyl-1,3,5-triazines that bear decyloxybenzene moieties. These compounds were synthesized by a Migita–Kosugi–Stille coupling reaction of 1,3,5-trichlorotriazine with three different tributyltin(thiazoles) as the key step. The structure–property relationships, namely the effects of the incorporation of thiazole units into the triazine unit, the conjugation connectivity between the thiazole and triazine units, and the insertion of ethynylene spacers between the thiazole and decyloxybenzene moieties on the properties of the trithiazolyl-1,3,5-triazines were comprehensively investigated. Binding of the triazine core at the 5-position of the thiazole moieties effectively extended the π-conjugation and afforded high fluorescence quantum yields. The ethynylene spacers substantially lowered the LUMO level relative to the HOMO level. The prepared trithiazolyl-1,3,5-triazines self-assembled in solution, and the introduction of thiazole units at the 5-position enhanced this behavior. Detailed thermodynamic studies on the self-association behavior were conducted, and the formation of self-assembled 1D clusters is disclosed.


 Please wait while we load your content...
Please wait while we load your content...