The carbon chain-selective adenylation enzyme TamA: the missing link between fatty acid and pyrrole natural product biosynthesis†
Abstract
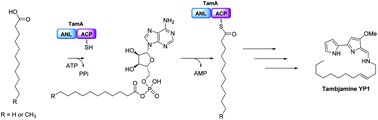
The marine bacterium Pseudoalteromonas tunicata produces the bipyrrole antibiotic tambjamine YP1. This natural product is built from common amino acid and fatty acid building blocks in a biosynthetic pathway that is encoded in the tam operon which contains 19 genes. The exact role that each of these Tam proteins plays in tambjamine biosynthesis is not known. Here, we provide evidence that TamA initiates the synthesis and controls the chain length of the essential tambjamine fatty amine tail. Sequence analysis suggests the unusual TamA is comprised of an N-terminal adenylation (ANL) domain fused to a C-terminal acyl carrier protein (ACP). Mass spectrometry analysis of recombinant TamA revealed the surprising presence of bound C11 and C12 acyl-adenylate intermediates. Acylation of the ACP domain was observed upon attachment of the phosphopantetheine (4′-PP) arm to the ACP. We also show that TamA can transfer fatty acids ranging in chain length from C6–C13 to an isolated ACP domain. Thus TamA bridges the gap between primary and secondary metabolism by linking fatty acid and pyrrole biosynthetic pathways.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...