Radiosynthesis of the anticancer nucleoside analogue Trifluridine using an automated 18F-trifluoromethylation procedure†
Abstract
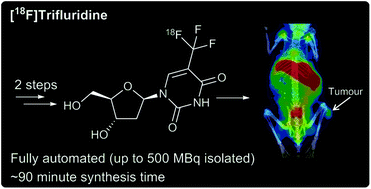
Trifluoromethyl groups are widespread in medicinal chemistry, yet there are limited 18F-radiochemistry techniques available for the production of the complementary PET agents. Herein, we report the first radiosynthesis of the anticancer nucleoside analogue trifluridine, using a fully automated, clinically-applicable 18F-trifluoromethylation procedure. [18F]Trifluridine was obtained after two synthetic steps in <2 hours. The isolated radiochemical yield was 3% ± 0.44 (n = 5), with a radiochemical purity >99%, and a molar activity of 0.4 GBq μmol−1 ± 0.05. Biodistribution and PET-imaging data using HCT116 tumour-bearing mice showed a 2.5 %ID g−1 tumour uptake of [18F]trifluridine at 60 minutes post-injection, with bone uptake becoming a prominent feature thereafter. In vivo metabolite analysis of selected tissues revealed the presence of the original radiolabelled nucleoside analogue, together with deglycosylated and phosphorylated [18F]trifluridine as the main metabolites. Our findings suggest a potential role for [18F]trifluridine as a PET radiotracer for elucidation of drug mechanism of action.


 Please wait while we load your content...
Please wait while we load your content...