Synthesis and aggregation behaviour of single-chain, 1,32-alkyl branched bis(phosphocholines): effect of lateral chain length†
Abstract
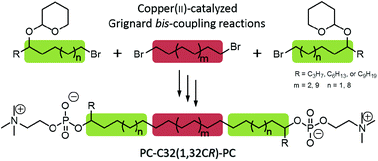
Three novel single-chain bis(phosphocholines) bearing two lateral alkyl chains of variable length next to the headgroup have been synthesized as model lipids for naturally occurring archaeal membrane lipids. The synthesis was realized using the Cu-catalyzed Grignard bis-coupling reaction of a primary bromide as a side part and a 1,ω-dibromide as a centre part. We could show that the aggregation behaviour of the resulting bolalipids strongly depends on the length of the lateral alkyl chain: the C3-branched bolalipid self-assembles into lamellar sheets, whereas the C6- and C9-analogues form nanofibres. The lamella-forming bolalipids could be used in the future to prepare stable and tailored liposomes for oral drug delivery.


 Please wait while we load your content...
Please wait while we load your content...