Secondary amine salt catalyzed controlled activation of 2-deoxy sugar lactols towards alpha-selective dehydrative glycosylation†
Abstract
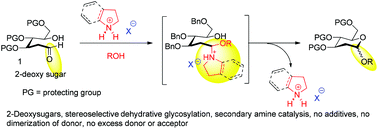
A new organocatalytic glycosylation method exploiting the lactol functionality has been disclosed. The catalytic generation of glycosyl oxacarbenium ions from lactols under forcible conditions via weakly Brønsted-acidic, readily available secondary amine salts affects the diastereoselective glycosylation of 2-deoxypyranoses and furanoses. This operationally simple iminium catalyzed activation of 2-deoxy hemi-acetals is a potential alternative to the existing cumbersome methods that need specialized handling. The mechanisms for this unique transformation and kinetic/thermodynamic effects have been discussed based on both experimental evidence and theoretical studies.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...