An aryl-fused redox-active tetrathiafulvalene with enhanced mixed-valence and radical-cation dimer stabilities†
Abstract
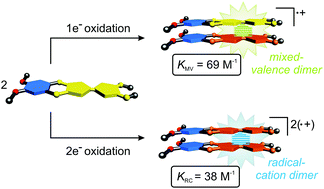
Molecular recognition of stable organic radicals is a relatively novel, but important structural binding motif in supramolecular chemistry. Here, we report on a redox-switchable veratrole-fused tetrathiafulvalene derivative VTTF which is ideally suited for this purpose and for the incorporation into stimuli-responsive systems. As revealed by electrochemistry, UV/Vis measurements, X-ray analysis, and electrocrystallisation, VTTF can be reversibly oxidised to the corresponding radical-cation or dication which shows optoelectronic and structural propterties similar to tetrathiafulvalene and tetrakis(methylthio)tetrathiafulvalene. However, theoretical calculations, variable temperature EPR, and NIR spectroscopy indicate that the dispersion-driven binding in the mixed-valence dimer (VTTF2)˙+ (KMV = 69 M−1 in CH2Cl2) and the radical-cation dimer (VTTF˙+)2 (KRC = 38 M−1 in CH3CN) is significantly enhanced by the additional veratrole π-surface in comparison to pristine tetrathiafulvalene.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...