Direct dihalo-alkoxylation of nitroalkenes leading to β,β-dihalo-β-nitroethyl alkyl ethers†
Abstract
A highly efficient one-pot synthesis of β,β-dihalo-β-nitroethyl alkyl ethers is achieved by the treatment of nitroalkenes with alcohols and N-halosuccinimides in the presence of sodium hydride. The notable advantages of this protocol are that it involves simple experimental manipulations and tolerates a wide range of functional groups. Further transformations of the obtained ethers, such as allylation and conversion to β,β-dihalogenated vinyl ethers, are also investigated.
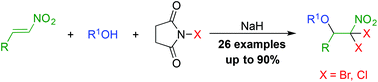


 Please wait while we load your content...
Please wait while we load your content...