Metal-free iodine(iii)-promoted synthesis of 2,5-diaryloxazoles†
Abstract
A nonmetal-catalyzed oxidative cyclization to achieve 2,5-disubstituted oxazoles from inexpensive and readily available substituted chalcone, (diacetoxyiodo)benzene (PIDA) and ammonium acetate (NH4OAc) at room temperature is described. The reaction forms a variety of 2,5-diaryloxazoles in good to excellent yields with broad substrate scope under mild conditions without the requirement of ligands and additional bases.
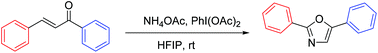
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...