First characterisation of two important postulated intermediates in the formation of a HydT DNA lesion, a thymidine oxidation product†
Abstract
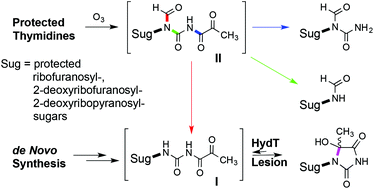
A number of environmental pollutants and endogenous oxidation agents form 1-(2-deoxy-β-D-ribofuranosyl)-5-hydroxy-5-methylhydantoin (HydT), an important DNA lesion resulting from thymidine oxidation. In this paper, two intermediates, postulated in the formation of HydT, have been characterised for the first time. The first, N1-formyl-N3-pyruvoylurea intermediate, was produced by the ozonolysis reaction of 2′,3′,5′-tri-O-acetylribo-, 3′,5′-di-O-TBS- and N3,O3′,O5-tribenzyl-protected thymidines and was shown to produce, upon decomposition and depending on the protecting group and the conditions, HydT alone, or together with protected-β-D-ribofuranosyl-N1-formylurea and formamide products. In addition, the second and long sought, open-chain-pyruvoylurea intermediate, was produced through de novo synthesis in protected β-D-ribofuranosyl-, 2-deoxy-β-D-ribofuranosyl- and 2-deoxy-β-D-ribopyranosyl systems. The conditions that induce the cyclization to the hydantoin ring of HydT have been determined. The chemistry utilised in the de novo synthesis is suitable for generating isotopically labelled HydT, as a reference in isotope-dilution-aided quantification of DNA damage.

 Please wait while we load your content...
Please wait while we load your content...