Direct oxidative C–H alkynylation of N-carbamoyl tetrahydroisoquinolines and dihydroisoquinolines†
Abstract
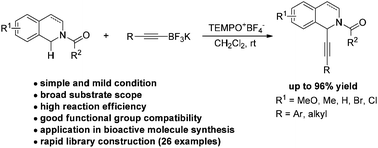
An efficient oxidative C–H alkynylation of N-carbamoyl tetrahydroisoquinolines mediated by a TEMPO oxoammonium salt has been established. A variety of electronically varied N-carbamoyl tetrahydroisoquinolines reacted with a range of alkynyl potassium trifluoroborates smoothly under mild metal-free conditions. Dihydroisoquinolines were also suitable components for the reaction. The synthetic applicability of the method for facile access to structurally diverse bioactive molecules was further demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...