Rh(iii)-Catalyzed regioselective mono- and di-iodination of azobenzenes using alkyl iodide†
Abstract
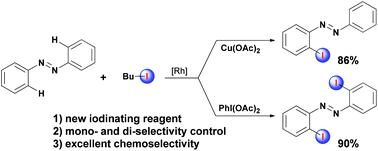
A new approach for highly regioselective iodination of azobenzenes with alkyl iodide as the iodinating reagent enabled by Rh-catalyzed oxidative C–H activation has been developed. By changing the oxidant, various mono- and di-iodinated azobenzenes were smoothly obtained in moderate to excellent yields, respectively. The preliminary mechanistic study reveals that the reaction process might undergo electrophilic substitution of the directed ortho metalated five-membered rhodacycle compound by an iodine cationic species generated in situ from alkyl iodide and oxidant.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...