Regioselective construction of 1,3-diaryl tetrahydroindazolones via the three-component reaction of 1,3-cyclohexanediones, β-nitrostyrenes and arylhydrazines†
Abstract
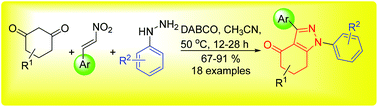
1,5,6,7-Tetrahydro-4H-indazol-4-one derivatives were successfully synthesized using a one-pot three-component system that combines substituted β-nitrostyrenes, 1,3-cyclohexanediones and phenylhydrazines. This reaction involves a highly efficient domino sequence consisting of the aza-Michael reaction, intramolecular O-nucleophilic addition, nucleophilic addition, and ring opening of furan as the key unit steps. Notably, the highly regioselective construction of the tetrahydro-4H-indazolone moiety and the introduction of functionalized aromatic rings were achieved.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...