Discovery of quinazoline-2,4(1H,3H)-dione derivatives as novel PARP-1/2 inhibitors: design, synthesis and their antitumor activity†
Abstract
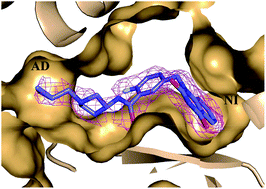
Novel quinazoline-2,4(1H,3H)-dione derivatives bearing a 3-amino pyrrolidine moiety were designed and synthesized as PARP-1/2 inhibitors. Structure–activity relationships were examined which revealed a number of potent PARP-1/2 inhibitors with moderate selectivity toward PARP-1 over PARP-2. These compounds had IC50 values against PARP-1 at the 10−9 M level and against PARP-2 at the 10−8 M level. Among all the synthesized compounds, compounds 10 and 11 displayed strong cytotoxicities which are either used as a single agent or in combination with temozolomide (TMZ) in MX-1 cells (10, IC50 < 3.12 μM, PF50 > 10; 11, IC50 = 3.02 μM, PF50 ≈ 10). In vivo tumor growth inhibition was investigated using compound 11 in combination with TMZ, and it was demonstrated that compound 11 could strongly potentiate the cytotoxicity of TMZ in a MX-1 xenograft tumor model. The co-crystal structure of compound 11 complexed with PARP-1 was achieved and demonstrated a unique binding mode.


 Please wait while we load your content...
Please wait while we load your content...