Super electron donor-mediated reductive transformation of nitrobenzenes: a novel strategy to synthesize azobenzenes and phenazines†
Abstract
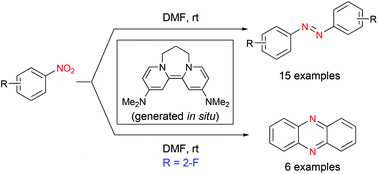
The transformation of nitrobenzenes into azobenzenes by pyridine-derived super electron donor 2 is described. This method provides an efficient synthesis of azobenzenes because of not requiring the use of expensive transition-metals, toxic or flammable reagents, or harsh conditions. Moreover, when using 2-fluoronitrobenzenes as substrates, phenazines were found to be obtained. The process affords a novel synthesis of phenazines.


 Please wait while we load your content...
Please wait while we load your content...