Copper-mediated regioselective hydrodifluoroalkylation of alkynes†
Abstract
A highly regioselective copper-mediated hydrodifluoroalkylation of alkynes with ethyl bromodifluoroacetate or bromodifluoroacetamides has been developed. This strategy provides straightforward access to a variety of difluoroalkylated alkenes under mild reaction conditions with low-cost cuprous bromide and metabisulfite as reduction agents. A wide range of alkynes are applicable under these reaction conditions. The excellent functional-group compatibility and good regio- and stereoselectivities are the notable features of this transformation.
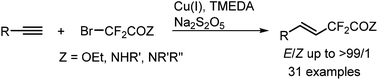
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...