Synthesis of thioureido peptidomimetics employing alkyl azides and dithiocarbamates†
Abstract
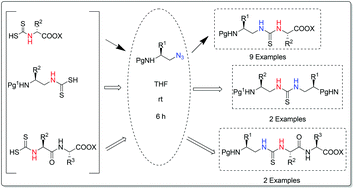
An unprecedented approach for the assembly of thioureido peptidomimetics is developed employing alkyl azides and dithiocarbamates. Dithiocarbamates react with alkyl azides with the liberation of N2 and elemental sulfur thereby leading to thiourea in a traceless manner. Thioureido peptidomimetics are thus furnished in good yields with no epimerization. This process is mild, free from the use of a base, scalable and step economic. The practicability of this methodology has been highlighted by the synthesis of N,N′-orthogonally protected thioureido peptidomimetics.
- This article is part of the themed collection: Chemical Biology in OBC
 Please wait while we load your content...
Please wait while we load your content...