A distinctive transformation based diversity oriented synthesis of small ring carbocycles and heterocycles from biocatalytically derived enantiopure α-substituted-β-hydroxyesters†
Abstract
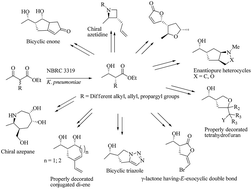
A series of structurally novel small ring carbocyclic and heterocyclic molecules were accessed in an enantiopure fashion. The starting materials, α-substituted-β-hydroxyesters, were achieved through the biocatalytic dynamic kinetic resolution of parent β-ketoesters in an excellent enantio and diastereocontrolled way. The active functional groups present in the starting precursor, were then tuned sequentially through certain key transformations (functional group interconversions; FGIs) to yield several small molecular scaffolds in a diverse way. Specific transformations such as halocyclization, ene–yne metathesis, dipolar cycloaddition, Mitsunobu cyclization, ring closing metathesis and Pauson–Khand reactions were mainly applied to generate the diversity.


 Please wait while we load your content...
Please wait while we load your content...