Temperature-modulated diastereoselective transformations of 2-vinylindoles to tetrahydrocarbazoles and tetrahydrocycloheptadiindoles†
Abstract
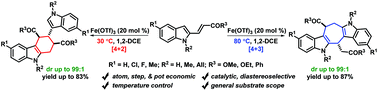
Direct and expedient access to densely substituted tetrahydrocarbazoles and tetrahydrocycloheptadiindoles bearing multiple contiguous stereocentres has been achieved via a two-fold divergent diastereoselective (dr up to >99 : 1) transformation of 2-vinylindoles. The high-yielding conversions (yield up to 87%) that are amenable for a wide range of substituted 2-vinylindoles proceed through Lewis acid-catalyzed [4 + 2] and [4 + 3] cyclization–aromatization cascade reactions, respectively, involving a heretofore-unprecedented reversal of the polarity (umpolung) of 2-vinylindoles. The two synthetic routes are effortlessly transposable into each other by merely modulating the temperature to furnish the corresponding products in a selective and exclusive fashion. In addition, another novel synthetic route to tetrahydroindolocarbazoles has been developed that advances via a formal [4 + 2] cyclization of 4-vinylindoles involving sequential C3 Michael addition–dearomatization–aromatization cascade reactions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...