Metal-free oxidative coupling of quinoxalin-2(1H)-ones with arylaldehydes leading to 3-acylated quinoxalin-2(1H)-ones†
Abstract
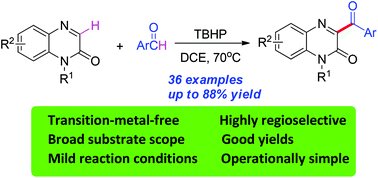
A facile TBHP-mediated direct oxidative coupling of quinoxalin-2(1H)-ones with arylaldehydes has been developed under metal-free conditions. This method provided a convenient and efficient approach to various 3-acylated quinoxalin-2(1H)-ones from readily available starting materials with excellent regioselectivity. This reaction proceeded efficiently under mild conditions over a broad range of substrates and with functional group tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...