Diastereoselective reduction of the tricarbonyl moiety in bicyclic tetramates giving pyroglutamates†
Abstract
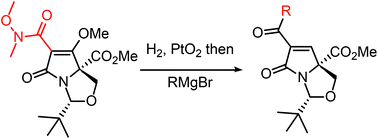
The reduction of C(6)-acyl bicyclic tetramic acids has been achieved with complete diastereoselectivity via catalytic hydrogenation using PtO2. The resulting pyroglutamates had potent antibacterial and anticancer properties and will allow the preparation of simple mimics of pyroglutamate-containing natural products.


 Please wait while we load your content...
Please wait while we load your content...