One-pot synthesis of thioesters with sodium thiosulfate as a sulfur surrogate under transition metal-free conditions†
Abstract
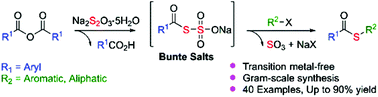
In this paper, we report an efficient synthetic method for thioester formation from sodium thiosulfate pentahydrate, organic halides, and aryl anhydrides. In the one-pot two-step reactions developed in this study, sodium thiosulfate was used as the sulfur surrogate for acylation with anhydrides, followed by substitution with organic halides through the in situ generation of thioaroylate. Furthermore, two important organic compounds could be successfully synthesized using our developed method. The advantages of the one-pot two-step reactions are operational simplicity, structurally diverse products with 42%–90% yields, use of relatively low toxic and odourless reagents, and easy applicability to large-scale operation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...