A copper/O2-mediated direct sp3 C–H/N–H cross-dehydrogen coupling reaction of acylated amines and N-aryl glycine esters†
Abstract
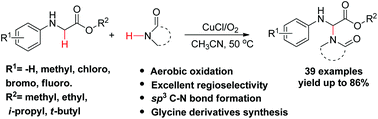
A copper salt-catalyzed cross-dehydrogenative coupling reaction between N-aryl glycine esters and acylated amines has been developed. The reaction proceeded effectively under an oxygen atmosphere without the use of peroxide agents. This simple protocol allows for the preparation of a series of new compounds in a moderate to excellent yield via the CDC reaction of a wide range of N-aryl glycine derivatives with acylated amines, which are of great interest in the field of medicinal chemistry. A plausible mechanism involving the formation of an iminium ion intermediate, followed by coupling with acylated amines was proposed after some control experiments were conducted.


 Please wait while we load your content...
Please wait while we load your content...