Fast, irreversible modification of cysteines through strain releasing conjugate additions of cyclopropenyl ketones†
Abstract
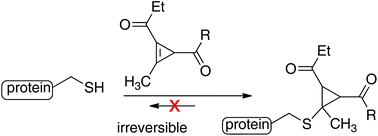
A method of cysteine alkylation using cyclopropenyl ketones is described. Due to the significant release of cyclopropene strain energy, reactions of thiols with cyclopropenyl ketones are both fast and irreversible and give rise to stable conjugate addition adducts. The resulting cyclopropenyl ketones have a low molecular weight and allow for simple attachment of amides via N-hydroxysuccinimide (NHS)-esters. While cyclopropenyl ketones do display slow background reactivity toward water, labeling by thiols is much more rapid. The reaction of a cyclopropenyl ketone with glutathione (GSH) proceeds with a rate of 595 M−1 s−1 in PBS at pH 7.4, which is considerably faster than α-halocarbonyl labeling reagents, and competitive with maleimide/thiol couplings. The method has been demonstrated in protein conjugation, and an arylthiolate conjugate was shown to be stable upon prolonged incubation in either GSH or human plasma. Finally, cyclopropenyl ketones were used to create PEG-based hydrogels that are stable to prolonged incubation in a reducing environment.


 Please wait while we load your content...
Please wait while we load your content...