New chiral amino alcohol ligands for catalytic enantioselective addition of diethylzincs to aldehydes†
Abstract
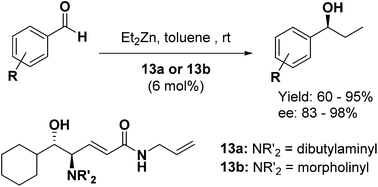
A study aimed at the synthesis and structure optimization of new, efficient, optically active β-amino alcohol ligands with a structure suitable for immobilization on magnetite nanoparticles has been carried out. The optimized homogeneous amino alcohol catalysts 13a and 13b, the chirality of which arises from the Sharpless epoxidation of suitable allyl alcohols, were tested by employing the well-established enantioselective amino alcohol-promoted addition of diethylzinc to benzaldehyde, giving the corresponding benzyl alcohol with nearly quantitative yield and ee = 95%. Then, their broad applicability as chiral catalysts was evaluated by carrying out the same reaction on a family of aldehydes, including variously substituted aromatic ones as well as an aliphatic analogue. The results have confirmed the validity of the fine-tuning process performed on ligands 13a and 13b. In fact, both exhibited excellent catalytic activity as demonstrated by the chemical yields and ee obtained from all the tested aldehydes, almost independent of the position and type of substitution in the aromatic ring.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...