Designing phenylalanine-based hybrid biological materials: controlling morphology via molecular composition†
Abstract
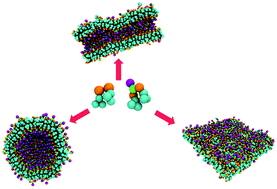
Harnessing the self-assembly of peptide sequences has demonstrated great promise in the domain of creating high precision shape-tunable biomaterials. The unique properties of peptides allow for a building block approach to material design. In this study, self-assembly of mixed systems encompassing two peptide sequences with identical hydrophobic regions and distinct polar segments is investigated. The two peptide sequences are diphenylalanine and phenylalanine-asparagine-phenylalanine. The study examines the impact of molecular composition (namely, the total peptide concentration and the relative tripeptide concentration) on the morphology of the self-assembled hybrid biological material. We report a rich polymorphism in the assemblies of these peptides and explain the relationship between the peptide sequence, concentration and the morphology of the supramolecular assembly.


 Please wait while we load your content...
Please wait while we load your content...
