Diarylethene-based xerogels: the fabrication of more entangled networks driven by isomerization and acidofluorochromism†
Abstract
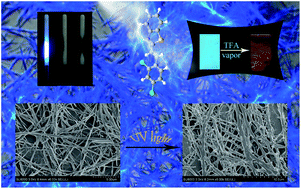
Fully-conjugated styrylbenzoxazoles and styrylbenzothiazoles of BOAF24, BOACl24, BOACl35, BOABr24, BOABr35, BTAF24, BTACl24 and BTABr24 without traditional gelation groups could form organogels. It was found that introduction of chlorine atoms in the 2,4-positions of the phenyl group would improve gelation abilities, and benzothiazole derivatives exhibited better gelation abilities than benzoxazoles with a similar π-skeleton due to better π-electron delocalization. Interestingly, the organogel of BTACl24 could change into solution by UV light due to trans–cis isomerization, which could also induce morphological changes in xerogels. The smooth organogel nanofibers stretched out lots of thin ‘arms’ to hold together or to catch other nanofibers upon UV irradiation, so more entangled networks were generated. Moreover, TFA (trifluoroacetic acid) could induce a gel–sol transformation on account of the protonation of the benzoxazole or benzothiazole unit, accompanied by emission quenching. BTACl24 exhibited higher performance than BOACl24 in the detection of TFA because of its strong basicity. The decay time and the detection limit of BTACl24 in xerogel-based film towards TFA vapor were of 0.7 s and 0.3 ppm, respectively. Therefore, organogelation of non-traditional organogelators is a powerful approach to the fabrication of multi-stimuli-responsive soft materials, and provides a new method to generate more entangled 3D networks through photochemical reactions in xerogels.


 Please wait while we load your content...
Please wait while we load your content...