Synthesis of indoles from aroyloxycarbamates with alkynes via decarboxylation/cyclization†
Abstract
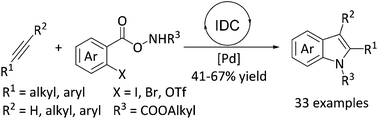
An efficient Pd-catalyzed decarboxylation/cyclization of aroyloxycarbamates to realize substituted indoles has been disclosed. Terminal alkynes as the coupling partners lead to site specific 2-substituted indoles through two pathways, while internal alkynes with aroyloxycarbamates can be transformed to 2,3-disubstituted indoles directly. This protocol is further demonstrated by the efficient synthesis of indoles as well as the success of employing inexpensive aryl acids as starting materials to construct C–N bonds by releasing CO2.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...