Metal-free synthesis of imidazole by BF3·Et2O promoted denitrogenative transannulation of N-sulfonyl-1,2,3-triazole†
Abstract
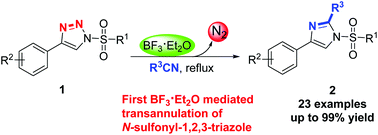
BF3·Et2O promoted metal-free denitrogenative transannulation of N-sulfonyl-1,2,3-triazole was reported. Rather than transition metals, BF3·Et2O was employed for the first time to promote the formation of α-diazoimines from N-sulfonyl-1,2,3-triazoles in nitriles, leading to the synthesis of various imidazoles. The protocol tolerates a broad range of functional groups and could also be applied to the late-stage modification of bioactive molecules, demonstrating the potential of this protocol in organic synthesis. A plausible mechanism was proposed.


 Please wait while we load your content...
Please wait while we load your content...