Computational evidence for a reaction pathway bifurcation in Sasaki-type (4 + 3)-cycloadditions†
Abstract
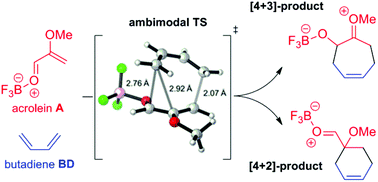
The current report seeks to validate the existence of a post-transition state bifurcation in the Lewis acid-catalysed (4 + 3)-cycloaddition of butadiene and α-methoxy acrolein. Cycloaddition transition state (TS) structures are shown by intrinsic reaction coordinate (IRC) and potential energy surface (PES) scan calculations to connect directly to both (4 + 3)- and (4 + 2)-products. A second TS, a 1,2-sigmatropic shift which interconverts the products, was also located. Implicit solvent is observed to have a substantial effect of the course of the reaction, with the minimum energy path from the gas phase TS leading to (4 + 2)-product whereas the DCM solvent phase TS leads to (4 + 3)-product. On the basis of these data it is suggested that a number of previously reported (4 + 3)-cycloadditions may also possess reaction pathway bifurcations.


 Please wait while we load your content...
Please wait while we load your content...