Organocatalytic asymmetric synthesis of benzazepinoindole derivatives with trifluoromethylated quaternary stereocenters by chiral phosphoric acid catalysts†
Abstract
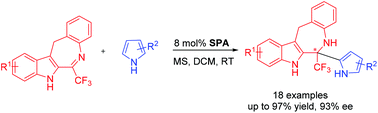
An enantioselective aza-Friedel–Crafts reaction of trifluoromethyl dihydrobenzoazepinoindoles with pyrroles catalyzed by a chiral spirocyclic phosphoric acid was developed. This methodology provides a facile route to CF3- and pyrrole-containing benzazepinoindoles bearing quaternary stereocenters in good yields and with moderate to excellent enantioselectivities (up to 93% ee). Indoles were also investigated as electron-rich aromatic substrates to afford the corresponding chiral heterocycles with good yields and considerable enantioselectivities. The introduction of CF3 shows a remarkable fluorine effect and increases the activation and stereoinduction.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...