Understanding the conformational analysis of gababutin based hybrid peptides†
Abstract
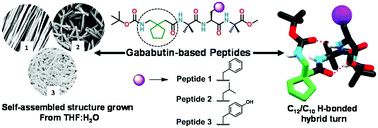
Constrained γ-amino acid gababutin (Gbn) based peptides that form different conformations have been synthesized. Striving to rationalize the impact of side chain orientations framing tetrapeptide-based supramolecular organic frameworks and morphological entities, Gbn incorporated hybrid peptides Boc-Gbn-Aib-Aaa-Aib-OMe (where Aaa = Phe(F) for peptide 1, Leu(L) for peptide 2 and Tyr(Y) for peptide 3) were synthesized by changing the amino acid at the third position. The solution state dual folded conformation (C12/C10 H-bonded) is probed by 2D NMR spectroscopy in support of a DMSO-d6 titration and VT NMR experiments. Peptides 1–3 adopt a C12/C10 type H-bonded dual folded conformation in the crystal state. In addition, distinct supramolecular frameworks result from the modification and orientation of the third residue side chain of peptides 1–3. A solvent induced morphological diversity of peptides 1–3 is attained by modifying the side chain backbone of the tetrapeptides, which are investigated by various microscopic (SEM and AFM) studies. Gbn-based peptides 1–3 show significant morphological and supramolecular packing properties, which are fairly different from those of their gabapentin (Gpn) based analogue peptides.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...