B(C6F5)3-catalyzed transfer hydrogenations of imines with Hantzsch esters†
Abstract
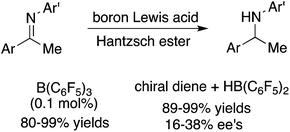
Highly efficient transfer hydrogenations of imines were realized with as low as 0.1 mol% of B(C6F5)3 by using Hantzsch esters as a hydrogen source, furnishing a variety of amines in 80–99% yields. For the asymmetric transfer hydrogenations, up to 38% ee was obtained with chiral diene-derived boron Lewis acids.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...