Direct C3-arylations of 2-indolylmethanols with tryptamines and tryptophols via an umpolung strategy†
Abstract
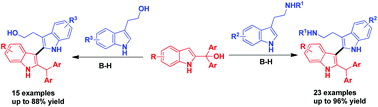
A Brønsted acid-catalyzed direct C3-arylation of 2-indolylmethanols with tryptamines and tryptophols has been established, leading to a series of potentially bioactive 2,3′-biindole derivatives with a broad substrate scope and generally good yields (38 examples, up to 96% yield). In this process, the reactivity of the C3-position of 2-indolylmethanol is switched from nucleophilic to electrophilic, which can serve as an umpolung strategy in indole chemistry. This protocol not only provides a new strategy for accessing structurally diversified 2,3′-biindolyl frameworks, but also satisfies the requirement of green chemistry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...