Total synthesis and structural elucidation of spongosoritin A†
Abstract
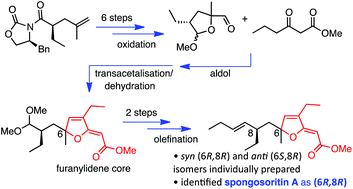
Two putative structures of spongosoritin A, with syn (6R,8R) and anti (6S,8R) configurations, were each synthesised in a total of 11 linear steps with only 8 purification procedures. The key steps in our strategy included Evans alkylation and olefin dihydroxylation to install the C8 and C6 stereocentres, a transacetalisation/dehydration cascade to construct the furanylidene core, and chromatographic separation of 9E- and 9Z-isomers of the final compounds with silver nitrate impregnated silica. Comparison of the 1H and 13C NMR data for the synthetic syn- and anti-isomers to that reported for the natural product revealed that the relative configuration of spongosoritin A is syn. The absolute stereochemistry was also confirmed as 6R,8R based on optical rotation measurements where the synthetic syn (6R,8R) and natural product had the same sign of optical rotation (negative).


 Please wait while we load your content...
Please wait while we load your content...