DBU-promoted carbonylative synthesis of 1,3-oxathiolan-2-ones from propargylic alcohols with TFBen as the CO source†
Abstract
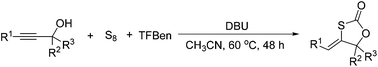
A DBU-promoted carbonylative cyclization of propargylic alcohols with sulfur was developed. Various 1,3-oxathiolan-2-ones were produced in 61–98% yields under mild conditions in the absence of metal catalysts. TFBen (benzene-1,3,5-triyl triformate) as an efficient and solid CO surrogate and S8 as an ideal sulfur source were employed and incorporated.


 Please wait while we load your content...
Please wait while we load your content...