Polysubstituted 3-trifluoromethylpyrazoles: regioselective (3 + 2)-cycloaddition of trifluoroacetonitrile imines with enol ethers and functional group transformations†
Abstract
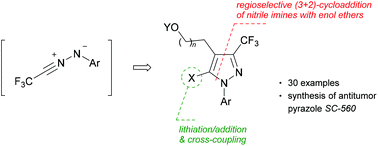
Non-catalysed addition of trifluoroacetonitrile imines to enol ethers provided fully regioselectively (3 + 2)-cycloadducts, which either spontaneously or via Brønsted acid-induced elimination of ROH molecules led to the formation of 3-trifluoromethylated pyrazoles. In the case of 2,3-dihydrofuran, the respective bicyclic intermediate was isolated and its structure was confirmed by X-ray analysis. Using the developed protocol the synthesis of a known antitumor compound SC-560 was performed in 45% yield. Subsequent functionalisations of selected 4-(ω-hydroxyalkyl)pyrazoles at C(5) through lithiation/addition, cross-coupling reactions or via intramolecular Pd-catalysed C–H arylations opened up an access to polysubstituted pyrazoles including unusual tricyclic systems comprising 7-membered rings (oxepane, thiepane and azepane) as the central unit.


 Please wait while we load your content...
Please wait while we load your content...