Disulphide bond exchange inhibited by air – kinetic and thermodynamic products in a library of macrocyclic cysteine derivatives†
Abstract
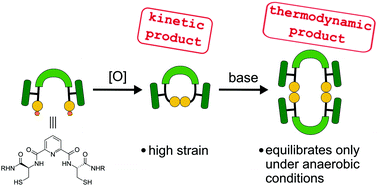
In this paper we present the synthesis and reactivity of dithiols comprising of two cysteine moieties attached to a dipicolinic acid core. Oxidation of these thiols provides oligomeric macrocycles. Monomers with 13-membered rings are kinetic products which are, however, strained and readily transform into higher oligomers under basic conditions or elevated temperature via a disulphide exchange reaction. Dimers, which are the most stable thermodynamic products, equilibrate only under inert conditions with thiolate as a catalyst. Under aerobic conditions, the thiols are oxidised before the equilibrium is reached.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...