NMR and experimental reinvestigation of the condensation reaction between γ-methylene-α,β-unsaturated aldehydes and propargyl aldehydes†
Abstract
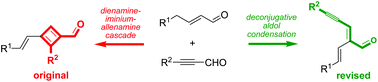
The condensation reaction between a γ-methylene-α,β-unsaturated aldehyde and phenylpropargyl aldehyde was revisited and, guided by extensive DFT calculations of NMR shifts, was found to afford a deconjugative aldol condensation product. In this manner, a simple protocol for the preparation of valuable cross-conjugated oxatrienes was uncovered.


 Please wait while we load your content...
Please wait while we load your content...