Divergent access to N-hydroxypyrroles and isoxazoles via the gold(i)- or Brønsted acid-catalysed regioselective cyclization of N-(2-trifluoromethyl-3-alkynyl) oximes†
Abstract
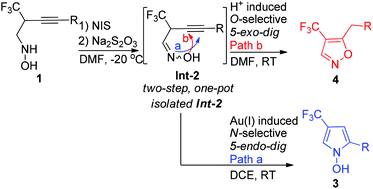
The divergent regioselective cyclization of N-(2-trifluoromethyl-3-alkynyl) oximes by a suitable choice of a gold(I) or a Brønsted acid catalyst, leading to 4-trifluoromethyl-N-hydroxypyrroles or 4-trifluoromethyl-5-alkylisoxazoles was developed. In order to avoid the tedious separation of unstable N-(2-trifluoromethyl-3-alkynyl) oximes, an easy two-step, one-pot synthesis of 4-trifluoromethyl-5-alkylisoxazoles was realized via the sequential oxidation of the corresponding hydroxylamines and subsequent treatment with the reductant, sodium thiosulfate (Na2S2O3). This two-step, one-pot procedure is a complementary method for the synthesis of 4-trifluoromethyl-5-alkylisoxazole from those unstable N-(2-trifluoromethyl-3-alkynyl) oximes.


 Please wait while we load your content...
Please wait while we load your content...