One-pot cascade synthesis of azabicycles via the nitro-Mannich reaction and N-alkylation†
Abstract
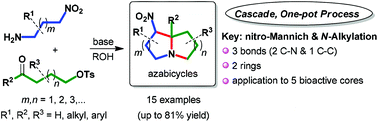
A one-pot, metal-free process for the synthesis of azabicycles is developed. The key transformations involved a cascade of double intramolecular cyclizations via the nitro-Mannich reaction and N-alkylation, providing various ring systems of azabicycles in yields up to 81% and an isomeric ratio of 62 : 1. This approach offers considerable advantages in terms of the handling of small molecules, the flexibility to introduce a functionalized side chain, and gives direct access to various azabicycles.


 Please wait while we load your content...
Please wait while we load your content...