Grignard-mediated rearrangement of trifluoroacetyl from dihydroisoquinoline enamides to afford tertiary trifluoromethylcarbinols†
Abstract
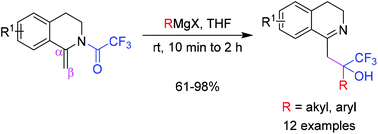
Treatment of the trifluoroacetyl enamides of dihydroisoquinolines 2 with diverse Grignard reagents afforded tertiary trifluoromethyl-carbinols 4 by facilitating the addition of tertiary carbinols to the β-carbon of enamides 2. Based on the confirmed formation of vinylogous amides 3, the transformation likely proceeds via unique acyl group rearrangement to the β-carbon of the enamide and subsequent nucleophilic addition of the Grignard reagent. Given the synthetic utility and novelty of this reaction, this work may open new avenues for the synthesis of pharmaceutically important tertiary trifluoromethylcarbinols on cyclic enamide systems.


 Please wait while we load your content...
Please wait while we load your content...