Eosin Y photoredox catalyzed net redox neutral reaction for regiospecific annulation to 3-sulfonylindoles via anion oxidation of sodium sulfinate salts†
Abstract
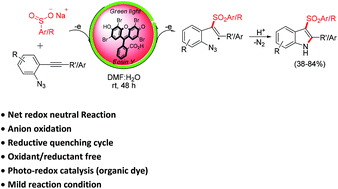
An eosin Y photoredox catalyzed net redox neutral process for 3-sulfonylindoles via the anionic oxidation of sodium sulfinate salts and its radical cascade cyclization with 2-alkynyl-azidoarenes was developed with visible light as a mediator. The reaction offers metal and oxidant/reductant free, visible light mediated vicinal sulfonamination of alkynes to 2-aryl/alkyl-3-sulfonylindoles and proceeds via the generation of a sulfur-centered radical through direct oxidation of the sulfinate anion by an excited photocatalyst with a reductive quenching cycle. The mild conditions, use of an organic dye as photo-catalyst, bench stability and easily accessible starting materials make the present approach green and attractive.

 Please wait while we load your content...
Please wait while we load your content...