Dissection of the effects that govern thioglucoside and thiomannoside reactivity†
Abstract
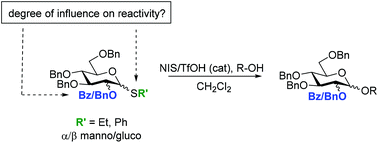
Neighboring group effects were investigated in gluco- and manno-configured thioglycosides under NIS/TfOH activation. Donors possessing a 2-O-benzoyl group that are capable (1,2-trans) and incapable (1,2-cis) of exerting nucleophilic push were compared with donors possessing a participatory neutral 2-O-benzyl group. By using competition experiments between sets of glycosyl donors the direct effect of neighboring group participation and the electron withdrawing effect of the 2-O-benzoyl group could be separated. The study brings insight into how the stereochemistry of the 1 and 2 position and how the nature of the aglycon (Ph or Et) have a pronounced effect on glycosyl donor reactivity.

 Please wait while we load your content...
Please wait while we load your content...