On the generality of the superarmament of glycosyl donors†
Abstract
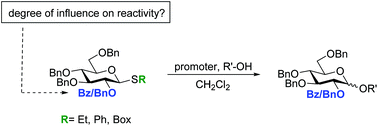
It was established that 2-O-benzoyl-3,4,6-tri-O-benzyl protected β-SEt, β-SPh and β-SBox glucosyl donors are not superarmed when using the NIS/TfOH promoter system, but instead have a similar reactivity as their classically armed tetra-O-benzyl protected glucosyl counterparts. The β-SBox 2-O-benzoyl-3,4,6-tri-O-benzyl glucosyl donor, however, was found to be superarmed under DMTST activation. Our studies have shown that the increased reactivity of the β-SBox 2-O-benzoyl-3,4,6-tri-O-benzyl glucosyl donor with DMTST activation could be a unique case, and that the high reactivity of glucosyl donors with the 2-O-benzoyl-3,4,6-tri-O-benzyl protection pattern is not general as earlier suggested.

 Please wait while we load your content...
Please wait while we load your content...