The Piancatelli reaction and its variants: recent applications to high added-value chemicals and biomass valorization
Abstract
The Piancatelli reaction, also called the Piancatelli rearrangement, consists in the direct conversion of furfuryl alcohols to cyclopentenone derivatives through a furan ring opening-electrocyclization process. Discovered in the late 70's, this reaction has been scarcely used for more than 40 years but recently has been the focus of particular interest from the scientific community and an increasing number of publications on the topic have emerged in the last few years. The first part of this review provides an overview of the recent achievements in classical Piancatelli reactions, discussing reaction conditions and catalytic systems, whereas the second part focuses on the variants recently developed, including the use of new nucleophiles in the process. Finally, the third part of this review deals with the recent application of this transformation to the production of commodity chemicals from renewable carbon feedstocks based on sugar-derived furanic platforms.
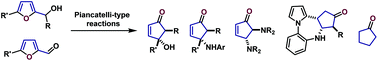
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...