Dess–Martin periodinane oxidative rearrangement for preparation of α-keto thioesters†
Abstract
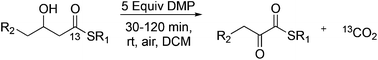
A Dess–Martin Periodinane (DMP) mediated oxidative rearrangement reaction was uncovered. The reaction proceeds via oxidation of a β-hydroxy thioester to a β-keto thioester, followed by an α-hydroxylation and then further oxidation to form a vicinal thioester tricarbonyl. This product then rearranges, extruding CO2, to form an α-keto product. The mechanism of the rearrangement was elucidated using 13C labelling and analysis of the intermediates as well as the products of the reaction. This efficient process allows for easy preparation of α-keto thioesters which are potential intermediates in the synthesis of pharmaceutically important heterocyclic scaffolds such as quinoxalinones.


 Please wait while we load your content...
Please wait while we load your content...