Catalyst-free synthesis of thiazolidines via sequential hydrolysis/rearrangement reactions of 5-arylidenethiazolidin-4-ones at room temperature†
Abstract
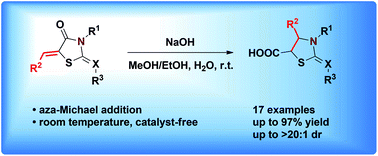
A catalyst-free sequential reaction involving hydrolysis and intramolecular aza-Michael addition was developed for synthesizing functionalized thiazolidines from 5-arylidenethiazolidin-4-ones at room temperature. A series of thiazolidine-5-carboxylic acids were prepared in good to excellent yields (up to 97% yield) and excellent diastereoselectivities (up to >20 : 1 dr). This methodology was applicable to the construction of derivatives of thiacloprid and flutianil with good yields.


 Please wait while we load your content...
Please wait while we load your content...