Synthesis of thiazolidine-thiones, imino-thiazolidines and oxazolidines via the base promoted cyclisation of epoxy-sulfonamides and heterocumulenes†
Abstract
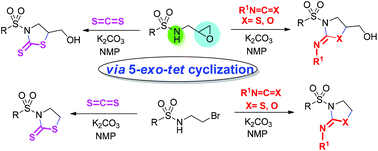
Epoxy-sulfonamides react with heterocumulenes (carbon disulfide/isothiocyanates/isocyanates) in the presence of a base to afford ring expansion products in good to high yields with excellent regioselectivity. N-(2-Bromoethyl)-sulfonamides can also be employed as substrates. This reaction proceeds through a 5-exo-tet pathway without forming aziridine intermediates.


 Please wait while we load your content...
Please wait while we load your content...