Teaching an old scaffold new recognition tricks: oligopyrrolamide antagonists of IAPP aggregation†
Abstract
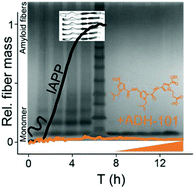
A library of N-substituted oligopyrrolamides was designed to modulate the aggregation kinetics of islet amyloid polypeptide (IAPP). IAPP is a hormonal peptide, co-secreted with insulin in the pancreatic β-cells. IAPP samples a variety of conformations, starting from a native random coil to membrane-associated α-helical intermediates and eventually terminates in the amyloid plaques rich in β-sheet structures. A growing body of evidence suggests that membrane bound α-helical intermediates are the key cytotoxic species that impair the functionality and viability of β-cells and contribute to the onset of type 2 diabetes mellitus (DM2). The N-substituted oligopyrrolamides were screened against the aggregation of IAPP using amyloid kinetic assays. A tripyrrole, ADH-101, was the most effective antagonist of IAPP fibrillation in a physiologically relevant lipid membrane system as well as under de novo conditions. ADH-101 induces/stabilizes a secondary structure in IAPP which potentially affects its downstream functions. ADH-101 efficiently affects IAPP-mediated liposome leakage and cell toxicity in insulin secreting cells. ADH-101 inhibits the elongation process potentially binding to the monomeric IAPP and attenuating its access to the preformed fibers. More importantly, oligopyrrolamides are better inhibitors of IAPP aggregation than analogous oligopyridylamides and have more desirable biological properties reflected by their partition coefficients. In essence, an oligopyrrolamide scaffold has been designed which modulates the membrane bound helical intermediates of IAPP and affects their downstream functions such as oligomerization, membrane poration, and cytotoxicity.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...